42 medication labels must include
A Guide To Veterinary Prescription Label Requirements ... What Is Required On A Veterinary Prescription Label As shown in the above example, the actual container must include the following information: The name of the veterinary practice, its address, and contact information The veterinarian's name, the patient's name and species, and the client's last name Guidance for Industry - Food and Drug Administration Minimize Medication Errors Additional copies are available from: Office of Communications . Division of Drug Information, WO51, Room 2201 . Center for Drug Evaluation and Research . Food and Drug ...
Get ready to assist clients with medication 2. Numbers on medication labels and documents Numbers are everywhere on medication labels and documents. The following medication label and document include examples of information the DSW needs to read and interpret. • Number of tablets • Batch number • Expiry date • Dosage • Age • Temperature • Pregnancy • Barcode ACTIVITY

Medication labels must include
4. Documenting Medications (MAR). | Aplmed Academy Each medication must be documented at the time of administration. For example, if eight medications are administered the QMAP must initial the MAR eight times indicating that each medication has been administered, refused or unavailable. New order: transcribe new medications on the MAR. Pharmacist FAQs - NCBOP The name must include, at a minimum, the first initial and full last name of the dispensing pharmacist. 11. If the dispensed drug is a "tranquilizer or sedative," it should bear the warning "The consumption of alcoholic beverages while on this medication can be harmful to your health" if the prescriber so directs on the prescription. Mixing Medications and Dietary Supplements Can Endanger ... Dietary supplements are widely used and include vitamins, minerals, and other less familiar substances—such as herbals, botanicals, amino acids, and …
Medication labels must include. Medicines: packaging, labelling and patient ... - GOV.UK Labels must include warnings for safe use of the medicine. All products that contain paracetamol must include statutory warnings. Additional warning statements must be included on the packaging of... FDA Says Drug Labels Must Include Clear Guidance for ... Drugs are currently labeled using one of five letters — A, B, C, D, and X. These letters indicate the overall risk of using a medication during pregnancy and breastfeeding. But even the FDA admits... FDA: Opioid, OUD Drug Labels Must Include Naloxone Co ... FDA will require drug makers to include naloxone co-prescribing information in labeling for all opioids and opioid use disorder medications, including buprenorphine, methadone and naltrexone, the agency announced Thursday (July 23) . What's on a prescription label? - Knowledge is the best ... * A Drug Identification Number (DIN) is an eight digit number assigned by Health Canada to a drug product prior to being marketed in Canada. It uniquely identifies all drug products sold in a dosage form in Canada and is located on the label of prescription and over-the-counter drug products that have been evaluated and authorized for sale in Canada.
Reading Medication Labels - Basicmedical Key Reading Medication Labels Objectives After reviewing this chapter, you should be able to identify: 1. The trade and generic names of medications 2. The dosage strength of medications 3. The form in which a medication is supplied 4. The total volume of a medication container where indicated 5. The Over-the-Counter Medicine Label: Take a Look | FDA All nonprescription, over-the-counter (OTC) medicine labels have detailed usage and warning information so consumers can properly choose and use the products. Below is an example of what the OTC... Prescription Drug Labeling Resources | FDA FDA-approved patient labeling [Medication Guides, Instructions for Use, and Patient Information (also called Patient Package Inserts)], and Carton and container labeling. The PI has two formats:... PDF Labeling on the Sterile Field: Improve Patient Safety and ... Labeling must include: Name of medication or solution, strength, date, and time Label one item at a time. Single items must also be labeled.
Ch 8 Pharmacy Flashcards & Practice Test - Quizlet A legal prescription label must include all of the following except: A. Directions for use B. Date the prescription was dispensed C. Name, address, and telephone number of the prescriber D. Name, address, and telephone number of the dispensing pharmacy Name, address, and telephone number of the prescriber Drug labeling, Information about Drug labeling - FAQs Each product must contain a label with "Supplement Facts" in bold letters onthe front panel. This is the manufacturer's opportunity to identify the product. Below "Supplement Facts," the panel must state the serving size. This isdetermined by the manufacturer with no input from the FDA. A prescription label must contain all of the following ... View full document. 92. A prescription label must contain all of the following, except: A. patient name B. patient addressC. patient ageD. name of the drug. 93. This consists of routine systematic review of the body: A. provisional diagnosis C. personal family history E. pathologic findings B. special examination D. physical examination. Chapter 8 NHA237 Flashcards - Quizlet If nothing is indicated on the prescription for refills, then the technician should: Check with the prescriber to see how many refills he or she wants. Tell the pharmacist. Enter the abbreviation, prn, (Latin, pro re nata) meaning as needed for refills. Enter zero refills. Enter zero refills.
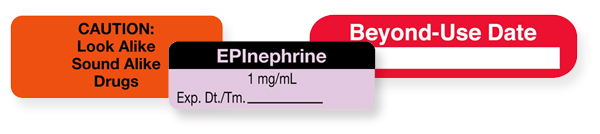
Safe Labeling Helps Prevent OR Medication Errors - OR Today Label information must include a medication's name and strength as well as amount when medications are mixed (as with antibiotic irrigations, tumescent and heparin solutions, and epinephrine). The unit of measure — percent, grams, milliliters, or units — must be recorded along with the date the medication is prepared.
How to read prescription drug labels - BeMedwise Navigating the "medicine label" of a prescription drug: How to read and understand the information in prescription labels Whenever you are prescribed a medication, you should read and follow the information in the medication's "label" in order to ensure your safety. All prescription medicine containers include information on the label including the patient's name, the […]
PDF Assistance With Self-administered Medications B. Prescription Labels Rx = Prescription: A written directive to a pharmacist giving names and quantities of ingredients to be combined and dispensed for a particular patient. 1. Prescription Label Prescription drug labels should be written according the doctor's order and should include: h Resident's name. h Name of the drug. h Strength of ...
How to Label Prescription Medication for Veterinary ... A label should include the following components: The name of the veterinary practice, its address, and contact information The veterinarian's name, the patient's name and species, and the client's...
PDF Chapter 20 Labeling Medications and Expiration Dating promotes medication safety a. Label the container if prepared but not administered immediately (no break in process) ... must also include patient name and location, directions for use, and auxiliary labels ... medications must be licensed by the FDA and the Florida Dept of Health as a
Pharmaceutical Labeling: Requirements & Guidelines To meet today's FDA regulations, labeling information on drugs must include the following in this order: - Product Name - Drug Facts Table - Active Ingredients - Purpose and Use - Warnings - Directions - Allergic Reactions - Inactive Ingredients
FDA says opioid labels must include information about naloxone W ASHINGTON — The Food and Drug Administration announced Thursday that it would require drug manufacturers to include information about naloxone, the overdose-reversal medication, on the labels ...
Over the Counter (OTC) Drug Labels - webPOISONCONTROL This is the easiest way to prevent errors and overdoses, because OTC medicines are often used without health professional advice. All over-the-counter (OTC) medication labels contain Drug Facts. Drug Facts include important information about the active ingredient (s), uses, warnings, doses, and directions. Serkalem Mekonnen, RN, BSN, MPH.
What Is a Drug Label? | The Motley Fool A drug label refers to all the printed information included with any dietary supplement, over-the-counter medicine, or prescription drug. They're strictly regulated by the Food and Drug...
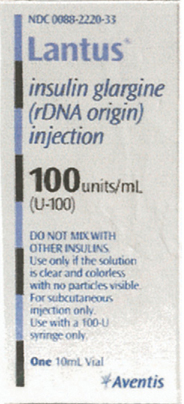
Understanding Drug Labels - Basicmedical Key • Identify brand and generic names on drug labels. • Identify dosage strengths of medications on drug labels. • Identify dosage forms and routes of administration on drug labels. • Identify total volume of drug containers and dosage supply of medications. • Define and identify NDC numbers and bar codes of drug products.
OTC Labeling Requirements - FindLaw (For example, drug products marketed under the Topical Antifungal Drug Products Monograph 2 should list their active ingredient's purpose as "Antifungal."). Below the "Drug Facts" section, a "Uses" section will list the approved or monograph indications for the drug. The next section of the label is "Warnings."
How to Read Over-the-Counter and Prescription Drug Labels Some labels include a seventh section with a phone number to call if you have questions or comments. The Drug Facts label for the over-the-counter drug acetaminophen, known by the brand name Tylenol, includes information about ingredients, uses, warnings and directions. Active Ingredient and Purpose.
McGuireWoods Consulting - Washington Healthcare Update FDA: Opioid, OUD Drug Labels Must Include Naloxone Co-Prescribing Info. On July 23, the Food and Drug Administration (FDA) announced that drug manufacturers to are required include naloxone co-prescribing information in labeling for all opioids and opioid use disorder (OUD) medications, including buprenorphine, methadone and naltrexone.
Mixing Medications and Dietary Supplements Can Endanger ... Dietary supplements are widely used and include vitamins, minerals, and other less familiar substances—such as herbals, botanicals, amino acids, and …
Pharmacist FAQs - NCBOP The name must include, at a minimum, the first initial and full last name of the dispensing pharmacist. 11. If the dispensed drug is a "tranquilizer or sedative," it should bear the warning "The consumption of alcoholic beverages while on this medication can be harmful to your health" if the prescriber so directs on the prescription.
4. Documenting Medications (MAR). | Aplmed Academy Each medication must be documented at the time of administration. For example, if eight medications are administered the QMAP must initial the MAR eight times indicating that each medication has been administered, refused or unavailable. New order: transcribe new medications on the MAR.











Post a Comment for "42 medication labels must include"