42 fda structured product labels
Structured Product Labeling Resources | FDA The Structured Product Labeling (SPL) is a document markup standard approved by Health Level Seven (HL7) and adopted by FDA as a mechanism for exchanging product and facility information. SPL... 2012AA FDA Structured Product Labels Source Information FDA Structured Product Label UNII Code for Active Substance: 9006: SHAPETEXT. FDA Structured Product Label imprint attribute for shape text: 8272: NDA. New Drug Application number for MTHSPL drug: 7563: OTC_MONOGRAPH_FINAL. FDA Structured Product Label OTC monograph status: 6843: OTC_MONOGRAPH_NOT_FINAL.
2010AB FDA Structured Product Labels Source Information FDA Structured Product Label UNII Code for Active Substance: 4093: COATING. FDA Structured Product Label imprint attribute for coating: 3034: SYMBOL. FDA Structured Product Label imprint attribute for symbol: 3032: DCSA. Controlled Substance Act designation code (e.g. 0,2,3n) 1975: NADA.

Fda structured product labels
FDA Label Search The device labeling has been reformatted to make it easier to read but its content has not been altered nor verified by FDA. The device labeling on this website may not be the labeling on currently... Structured Product Labeling - Wikipedia Structured Product Labeling ( SPL) is a Health Level Seven International (HL7) standard which defines the content of human prescription drug labeling in an XML format. The "drug labeling" includes all published material accompanying a drug, such as the Prescribing Information which contains a great deal of detailed information about the drug. Cannabis Strain Labels Are Misleading, Research Suggests: Authors Call ... The research found that "commercial labels do not consistently align with the observed chemical diversity," of cannabis products. This compelled the authors to call for a labeling system ...
Fda structured product labels. DailyMed The National Library of Medicine (NLM)'s DailyMed searchable database provides the most recent labeling submitted to the Food and Drug Administration (FDA) by companies and currently in use (i.e., "in use" labeling). DailyMed contains labeling for prescription and nonprescription drugs for human and animal use, and for additional products such as medical gases, devices, cosmetics, dietary ... FDA SPL - Structured Product & Drug Labeling Composition Process | Reed ... Structured Product Labeling (SPL) is a Health Level Seven (HL7) International standard for regulatory guidance documents as a method for communicating product and facility information. Accepted by the Food and Drug Administration, structured product labeling enhances the cohesiveness and honesty of product information because it requires ... Assessing the Impact of HL7/FDA Structured Product Label (SPL) Content ... The amount and quality of the SPL drug knowledge which has been released so far is assessed. All published labels were loaded into a relational database and classified to create vendor-independent descriptions. While SPL labels cover only 23% of RxNorm ... DailyMed - FDA Resources: SPL, Other Prescription Drug Labeling ... FDA's Structured Product Labeling Resources. Structured Product Labeling (SPL) is the standard format for electronic submission of the content of labeling. For SPL resources (including industry data standards for SPL), see FDA's SPL Resources page and the "Structured Product Labeling Resources" heading on FDA's Prescription Drug Labeling ...
Package Type | FDA In this section: Structured Product Labeling Resources ... Electronic Animal Drug Product Listing Directory; Equivalence Codes; Flavor; Geopolitical Entities, Names, and Codes (GENC) ... Nsde | Fda CMS Memo - PDE Editing using the FDA Online Label Repository (PDF) With the exception of the billing unit data in the NSDE document, this file is generated from SPL documents sent to FDA for... Structured Product Labeling Validation Rules Guidance for Industry - Indexing Structured Product Labeling (Final) Guidance for Industry: Self-Identification of Generic Drug Facilities, Sites, and Organizations ... 4 Drug Labeling, Listing ... FDA Label Search-Company Name 10903 New Hampshire Avenue. Silver Spring, MD 20993. Ph. 1-888-INFO-FDA (1-888-463-6332) Contact FDA. For Government. For Press.
2012AB FDA Structured Product Labels Source Information FDA Structured Product Label SET_ID code. 459423. NDC. National Drug Code corresponding to a clinical drug (e.g. 000023082503) 144687. DM_SPL_ID. DailyMed internal identifier for MTHSPL atom. 70092. LABELER. IIS COVID-19 Vaccine Related Code | CDC The following vaccines and associated tradenames have been approved by the FDA under BLA License. They are listed separately because while they may represent the same formulations as the EUA authorized and labeled products listed above, the NDCs listed with the new BLA licensed tradenames in the FDA BLA approval or the FDA Structured Product Labels (SPL) are not currently being produced by the ... Comirnaty Not Available - by Warner Mendenhall NDCs are listed per FDA Structured Product Label (SPL) document for the BLA licensed product. ... Pfizer has provided the following statement regarding the COMINARTY branded NDCs and labels: "Pfizer received FDA BLA license on 8/23/2021 for its COVID-19 vaccine for use in individuals 16 and older (COMIRNATY). At that time, the FDA published a ... SPL for FDA Submission - Dakota Systems Through the use of a standard, structured format, measurable improvements can be achieved throughout the creation, review, approval and overall management and distribution of labeling content by both industry and health authorities. ... SPL Challenges and FDA Compliance. Product labeling is a highly regulated and complex process. The product ...
MTHSPL (FDA Structured Product Labels) - Statistics FDA Structured Product Label imprint attribute for shape text: 18077: BLA: Therapeutic Biologic Applications number for the MTHSPL drug: 15324: NDA: New Drug Application number for MTHSPL drug: 11751: DCSA: Controlled Substance Act designation code (e.g. 0,2,3n) 7193: MARKETING_EFFECTIVE_TIME_HIGH:
Understanding FDA Food Labeling: A Guide for Food Manufacturers The full footnote details the FDA recommended daily values for specific nutrients and is required if the product packaging uses more than 40 square inches for labeling. The footnote must include: The following statement: "Percent Daily Values are based on a 2,000 calorie diet. Your daily values may be higher or.
DrugBank Admin DrugBank Help Center
SPL Xforms | FDA To register, please submit the following information via e-mail to spl@fda.hhs.gov: Attendee's first and last name. Name of your organization. E-mail address. Session name and date of training ...
2009AB FDA Structured Product Labels Source Information Drug labels and the most recently updated information are available on DailyMed. Health information suppliers can download drug labels in SPL format from DailyMed. Drug labeling on DailyMed is the most recent submitted to the FDA and currently in use. Sites Consulted. FDA Data Standards Council. Rockville (MD): Food and Drug Administration (US).
Indexing Structured Product Labeling | FDA Center for Drug Evaluation and Research Center for Biologics Evaluation and Research This guidance explains that FDA's Center for Drug Evaluation and Research (CDER) and Center for Biologics...
Structured Product Labeling - Food and Drug Administration Structured Product Labeling (SPL) is a document markup standard approved by Health Level Seven (HL7) and adopted by FDA as a mechanism for exchanging product and facility information. The openFDA...
Drug Labeling Overview - Food and Drug Administration Drug manufacturers and distributors submit documentation about their products to FDA in the Structured Product Labeling (SPL) format. The openFDA drug product labeling API returns data from this...
FDALabel: Full-Text Search of Drug Product Labeling | FDA The FDALabel Database is a web-based application used to perform customizable searches of over 140,000 human prescription, biological, over-the-counter (OTC), and animal drug labeling documents....
MTHSPL (FDA Structured Product Labeling) Source Information AuthorityThe U.S. National Library of Medicine (NLM) produces the Metathesaurus FDA Structured Product Labels (MTHSPL), which is based on the Food and Drug Administration (FDA) Structured Product Labeling (SPL). Information for this source is extracted from the NLM DailyMed Web site.
2011AA FDA Structured Product Labels Source Information IMPRINT_CODE. FDA Structured Product Label imprint attribute for code. 14314. ANDA. Abbreviated New (Generic) Drug application number for the MTHSPL drug. 11049. BLA. Therapeutic Biologic Applications number for the MTHSPL drug.
UMLS Metathesaurus - MTHSPL (FDA Structured Product Labels) - Synopsis Authority The U.S. National Library of Medicine (NLM) produces the Metathesaurus FDA Structured Product Labels (MTHSPL), which is based on the Food and Drug Administration (FDA) Structured Product Labeling (SPL). Information for this source is extracted from the NLM DailyMed Web site. Purpose
Cannabis Strain Labels Are Misleading, Research Suggests: Authors Call ... The research found that "commercial labels do not consistently align with the observed chemical diversity," of cannabis products. This compelled the authors to call for a labeling system ...
Structured Product Labeling - Wikipedia Structured Product Labeling ( SPL) is a Health Level Seven International (HL7) standard which defines the content of human prescription drug labeling in an XML format. The "drug labeling" includes all published material accompanying a drug, such as the Prescribing Information which contains a great deal of detailed information about the drug.
FDA Label Search The device labeling has been reformatted to make it easier to read but its content has not been altered nor verified by FDA. The device labeling on this website may not be the labeling on currently...



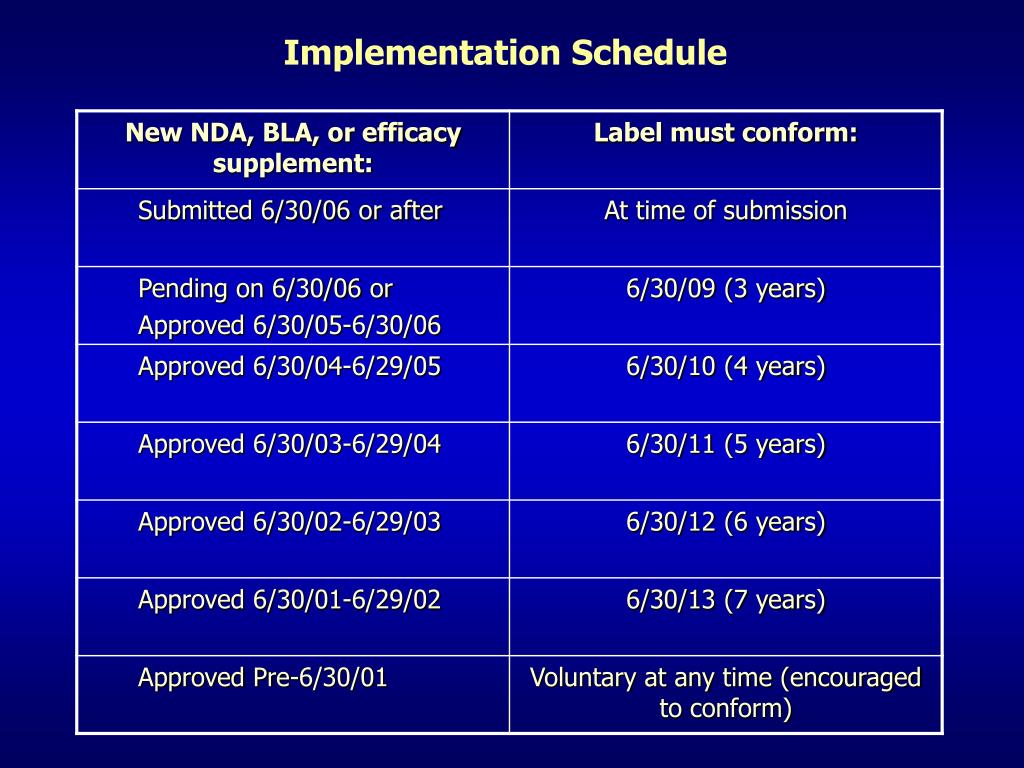

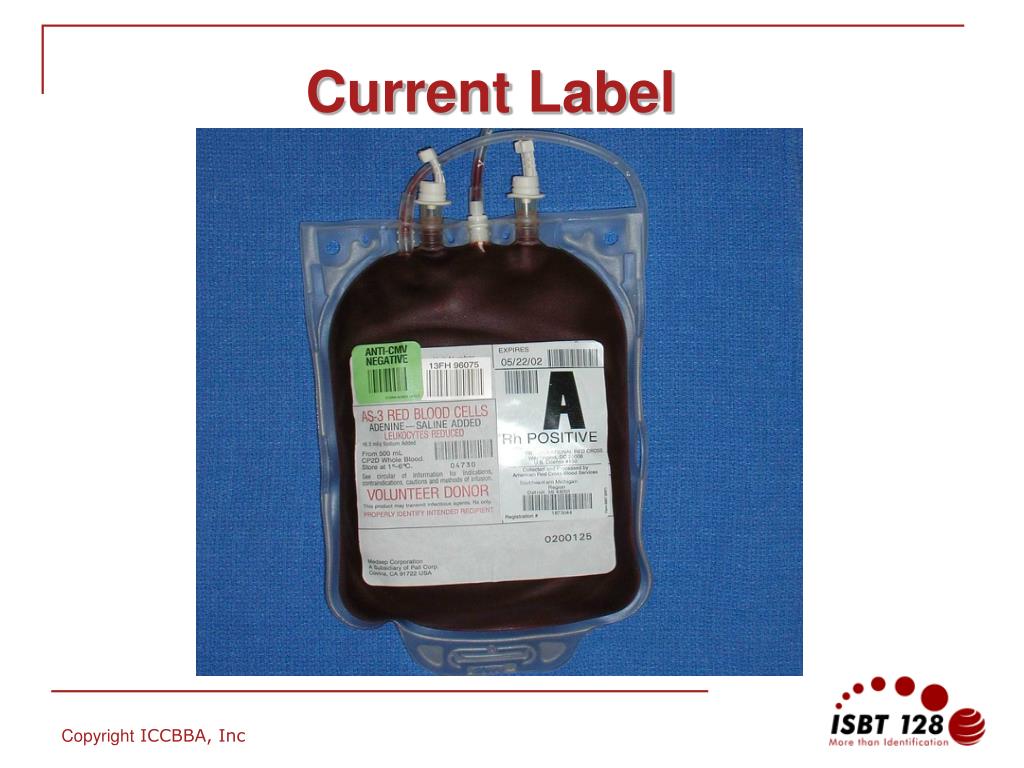





Post a Comment for "42 fda structured product labels"